Specifications
- Purity
- ≥97% (NMR)
- Appearance
- Yellow powder
- Identity
- 1H-NMR
Properties
- Solvents
- chloroform
- Melting Point
- 212-215 °C
- Fluorescence
- λex 272 nm in methanol, λex 490, λem 514 (Reaction product)
Downloads
- Safety Data Sheet
- CDX D0122 MSDS.pdf
Category: Fluorescent Detection, Fluorescent Detection
Dihydrofluorescein diacetate is a fluorescent probe for detecting intracellular oxidants. It is hydrolyzed by cellular esterases to dihydrofluorescein and is then oxidized to fluorescein primarily by H2O2. Dihydrofluorescein diacetate might be reactive toward a broad range of oxidizing reactions that may be increased during intracellular oxidant stress. Cell-loading studies indicate that dihydrofluorescein achieves higher intracellular concentrations than the other redox sensors such as Dihydrorhodamine 123 (Ex/Em: 490/514nm).
| Synonyms | Diacetyldihydrofluorescein |
|---|---|
| Purity | ≥97% (NMR) |
| Appearance | Yellow powder |
| CAS-Number | 35340-49-9 |
| Molecular Formula | C24H18O7 |
| Molecular Weight | 418.4 |
| Identity | 1H-NMR |
| Solvents | chloroform |
| Melting Point | 212-215 °C |
| Fluorescence | λex 272 nm in methanol, λex 490, λem 514 (Reaction product) |
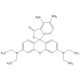
| Smiles | CC(=O)OC1=CC2=C(C=C1)C(C1=C(O2)C=C(OC(C)=O)C=C1)C1=C(C=CC=C1)C(O)=O |
| InChi Key | YKSJJXGQHSESKB-UHFFFAOYSA-N |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use / Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Transportation | Not dangerous goods |
| References | (1) P.P. Williams & R.L. Stolzenberg: Applied Microbiology 23(4), 745-9 (1972), (2) S.L. Hempel, et al.: Free Radical Biology & Medicine 27(1/2), 146-159 (1999), (3) Y. O'Malley et al.: Free Radical Biology & Medicine 36(1), 90-100 (2004) |
| InChi | InChI=1S/C24H18O7/c1-13(25)29-15-7-9-19-21(11-15)31-22-12-16(30-14(2)26)8-10-20(22)23(19)17-5-3-4-6-18(17)24(27)28/h3-12,23H,1-2H3,(H,27,28) |
| Quantity | 1 g, 10 g, Bulk |
 2,3-Diaminopropane-1-thiol dihydrobromide
2,3-Diaminopropane-1-thiol dihydrobromide