| References |
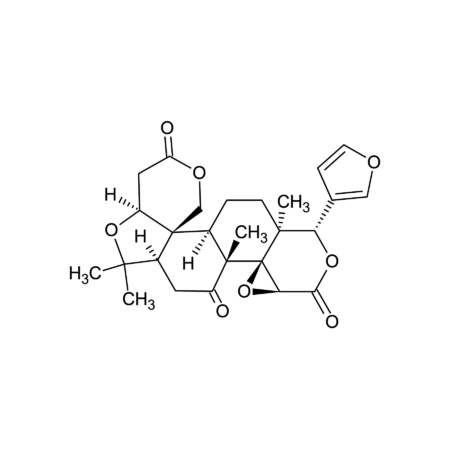
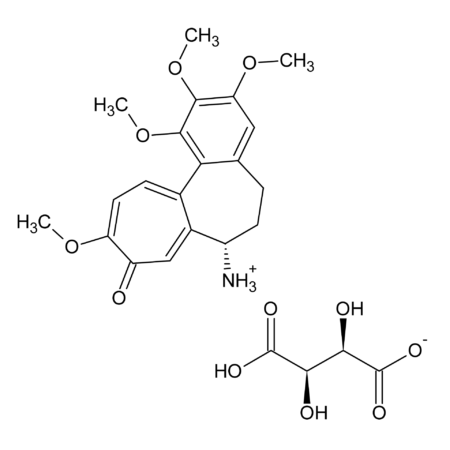
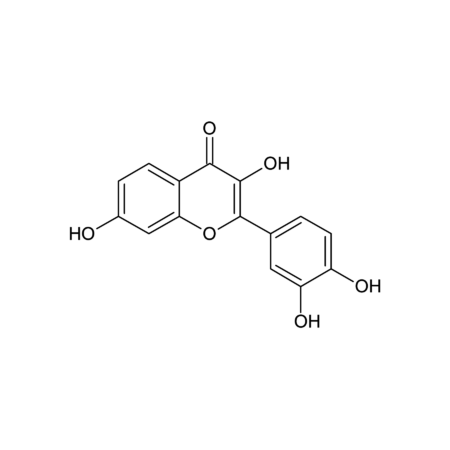
(1) R. Pompei, et al., Experientia 36, 304 (1980), (2) N.M. Gandhi, et al., J. Radiat. Res. 45, 461 (2004), (3) C.Y. Wang, et al., J. Agric. Food Chem. 59, 7726 (2011), (4) Y.Y. Chia, et al., Eur. J. Pharmacol. 677, 197 (2012), (5) K.J. Kim, et al., Phytother. Res. 27, 841 (2013), (6) F.S. Chueh, et al., Oncol. Rep. 28, 2069 (2012), (7) E.S. Ramli, et al., J. Bone Miner. Metab. 31, 262 (2013), (8) L.J. Ming & A.C. Yin, Nat. Prod. Commun. 8, 415 (2013) (Review), (9) A. Lau, et al., Am. J. Nephrol. 40, 84 (2014), (10) H.S. Cheng, et al., Nat. Prod. Bioprospect. 4, 325 (2014), (11) Y. Ohtsu, et al., Mol. Cell. Endocrinol. 394, 70 (2014), (12) L. Wang, et al., Acta Pharmac. Sin. B 5, 310 (2015), (13) S. Ojha, et al., Neurotox. Res. 29, 275 (2016), (14) L.Y. Wang, et al., Biomed. Pharmacother. 95, 599 (2017), (15) Z.G. Sun, et al., Mini Rev. Med. Chem. 19, 826 (2019) (Review), (16) O.Y. Selyutina & N.E. Polyakov, Int. J. Pharm. 559, 271 (2019) (Review), (17) Y. Yoshida, et al., Sci. Rep. 9, 10243 (2019)
|
| InChi |
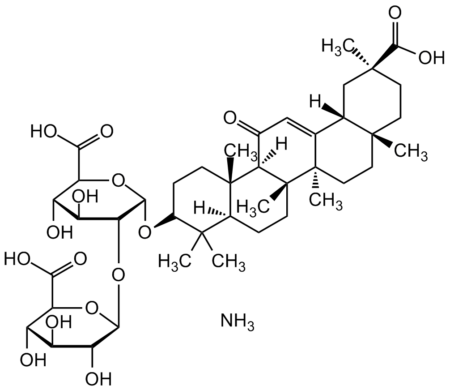
InChI=1S/C42H62O16.H3N/c1-37(2)21-8-11-42(7)31(20(43)16-18-19-17-39(4,36(53)54)13-12-38(19,3)14-15-41(18,42)6)40(21,5)10-9-22(37)55-35-30(26(47)25(46)29(57-35)33(51)52)58-34-27(48)23(44)24(45)28(56-34)32(49)50,/h16,19,21-31,34-35,44-48H,8-15,17H2,1-7H3,(H,49,50)(H,51,52)(H,53,54),1H3/t19-,21-,22+,23-,24-,25-,26-,27+,28-,29-,30+,31+,34-,35-,38+,39-,40-,41+,42+,/m0./s1
|