Specifications
- Purity
- ≥95% (HPLC)
- Appearance
- Light yellow powder
Properties
- Solvents
- DMSO, pyridine
- Melting Point
- 195°C
Downloads
- Safety Data Sheet
- CDX R0066 MSDS.pdf
Category: Phytochemicals
A polyphenolic flavonoid that acts as an antioxidant and NO scavenger. It can attenuate peroxide production in glial cells by acting as a free radical scavenger and protect renal cells from oxidative injury. Inclusion of rutin in the diet of rats significantly reduced the appearance of single-strand breaks in nuclear DNA caused by hepatocarcinogens aflatoxin B1 and N-nitrosodiumethylamine. This protection from DNA damage was found to be due to a reduction in the induction of repair enzymes polymerase, DNA polymerase ? and DNA ligase. Since DNA damage and inefficient repair are thought to initiate the process of carcinogenesis, effects of rutin on these functions suggests a protective role of this flavonoid against carcinogenesis induced by chemical carcinogens. Rutin ameliorates obesity through brown fat activation.
| Synonyms | Rutoside, Phytomelin, Sophorin, Birutan, Eldrin, Birutan Forte, Globularicitrin, Violaquercitrin, Quercetin rutinoside |
|---|---|
| Purity | ≥95% (HPLC) |
| Appearance | Light yellow powder |
| CAS-Number | 250249-75-3 |
| Molecular Formula | C27H30O16 |
| Molecular Weight | 610.52 |
| Solvents | DMSO, pyridine |
| Melting Point | 195°C |
| Smiles | O=C1C2=C(O)C=C(O)C=C2OC(C3=CC(O)=C(O)C=C3)=C1O[C@H]4[C@H](O)[C@@H](O)[C@H](O)[C@@H](CO[C@H]5[C@H](O)[C@H](O)[C@@H](O)C(C)O5)O4 |
| InChi Key | IKGXIBQEEMLURG-KAOWGYSOSA-N |
| Shipping | AMBIENT |
| Short Term Storage | RT |
| Long Term Storage | RT |
| Handling Advice | Protect from light and moisture. |
| Use / Stability | Stable for at least 2 years after receipt when stored at RT. |
| Hazard statements | H302 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| GHS Symbol | GHS07 |
| Signal word | Warning |
| Transportation | Not dangerous goods |
| References | I.B. Afanas'ev, et al., Biochem. Pharmacol. 38, 1763 (1989), R.P. Webster, et al., Cancer Lett. 109, 185 (1996), E.A. Ostrakhovitch & I.B. Afanas'ev, Biochem. Pharmacol. 62, 743 (2001), N. Kamalakkannan & P.S. Prince, Basic Clin. Pharmacol. Toxicol. 98, 97 (2006), J.P. Lin, et al., Leuk. Res. 33, 823 (2009), R. Lapa Fda, et al., J. Pharm. Pharmacol. 63, 875 (2011), S.W. Wang, et al., Neurotoxicology 33, 482 (2012), X. Yuan, et al., FASEB J. 31, 333 (2016) |
| InChi | InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-33,35-38H,7H2,1H3/t8?,15-,17+,18-,20-,21+,22-,23-,26-,27+/m1/s1 |
| Quantity | 100 g, 500 g, Bulk |
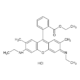
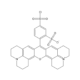 Sulforhodamine 101 acid chloride
Sulforhodamine 101 acid chloride