AMCA-H
- SKU
- A0009
Category: Fluorescent Detection, Fluorescent Detection
- Synonyms
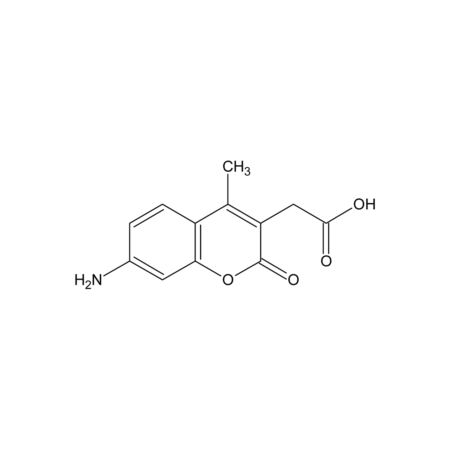
- 7-Amino-4-methyl-3-coumarinylacetic acid
- 106562-32-7
- CAS-Number
- C12H11NO4
- Molecular Formula
- 233.22
- Molecular Weight
Specifications
- Purity
- ≥90% (HPLC)
- Appearance
- Brown solid
- Identity
- 1H-NMR
Properties
- Solvents
- DMSO, DMF
- Fluorescence
- λex 350 nm, λem 443 nm in methanol
Downloads
- Safety Data Sheet
- CDX A0009 MSDS.pdf
- Certificate of Analysis
- CDX A0009 CofA N6023_Vs3.PDF
- Specification
- CDX A0009 Specifications.pdf
- 1H-NMR
- CDX A0009 NMR N6023.pdf
- Shipping
- AMBIENT
- Short Term Storage
- +4°C
- Long Term Storage
- -20°C
- Handling Advice
- Protect from light and moisture.
- Use / Stability
- Stable for at least 2 years after receipt when stored at -20°C.
- Hazard statements
- H315, H319, H335
- Precautionary statements
- P261, P305 + P351 + P338
- GHS Symbol
- GHS07
- Signal word
- Warning
- Transportation
- Not dangerous goods
- Description
- AMCA is one of the brightest amine-reactive blue fluorescent dyes useful for immunofluorescence and fluorescent labeling (Ex/Em: 353/455nm). It is quite photostable, and its fluorescence is pH-independent from pH 4 to 10. The properties include a relatively large Stoke's shift and resistance to photobleaching. Reactive AMCA derivatives are used as contrasting probes for double and triple labeling in immunofluorescence microscopy, arrays and in situ hybridization. NHS-AMCA and Sulfo-NHS-AMCA are reactive towards primary amine groups on antibodies, proteins, peptides and other biomolecules. AMCA-Hydrazide is used to label glycosylation sites or for conjugation to carboxyl groups using the crosslinker EDC.
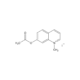
- Smiles
- CC1=C(CC(O)=O)C(=O)OC2=C1C=CC(N)=C2
- InChi Key
- QEQDLKUMPUDNPG-UHFFFAOYSA-N
- References
- (1) G-L. Ferri et al.: J. Histochem. Cytochem. 45(2), 155 (1997) , (2) B. Ufhake et al.: J. Neurosc. Meth. 40(1), 39 (1991) , (3) M. W. Wessendorf et al.: J. Histochem. Cytochem. 38(1), 87 (1990)
- InChi
- InChI=1S/C12H11NO4/c1-6-8-3-2-7(13)4-10(8)17-12(16)9(6)5-11(14)15/h2-4H,5,13H2,1H3,(H,14,15)
AMCA is one of the brightest amine-reactive blue fluorescent dyes useful for immunofluorescence and fluorescent labeling (Ex/Em: 353/455nm). It is quite photostable, and its fluorescence is pH-independent from pH 4 to 10. The properties include a relatively large Stoke’s shift and resistance to photobleaching. Reactive AMCA derivatives are used as contrasting probes for double and triple labeling in immunofluorescence microscopy, arrays and in situ hybridization. NHS-AMCA and Sulfo-NHS-AMCA are reactive towards primary amine groups on antibodies, proteins, peptides and other biomolecules. AMCA-Hydrazide is used to label glycosylation sites or for conjugation to carboxyl groups using the crosslinker EDC.