| References |
(1) M. Bodanszky & D.T. Fagan, JACS 99, 235 (1977), (2) E. Friauf, et al., J. Neurosci. 10, 2601 (1990), (3) X.J. Sun, et al., J. Histochem. Cytochem. 46, 263 (1998), (4) A. Erisir & C. Aoki, J. Neurosci. Methods 81, 189 (1998), (5) C. Koebbert, et al, Prog. Neurobiol. 62, 327 (2000), (6) S.L. Chang, et al., J. Neurosci. Methods 97, 1 (2000), (7) K. Kumasaka, et al., Clin. Chim. Acta 306, 71 (2001), (8) Y. Morozov, et al., J. Neurosci. Methods 117, 81 (2002), (9) G. Roth, et al., J. Comp. Neurol. 478, 35 (2004), (10) H.G. Xue, et al., Brain Res. Brain Res. Protoc. 13, 106 (2004), (11) M.A. MacNeil & P.A. Gaul, J. Comp. Neurol. 506, 6 (2008), (12) R. Bopp, et al., PLoS Biol. 12, 1 (2014)
|
| InChi |
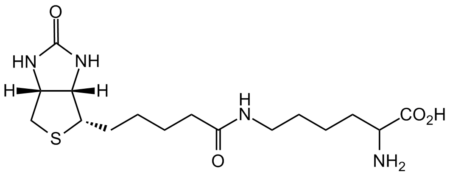
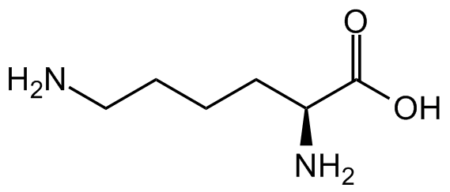
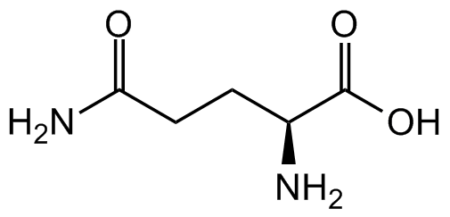
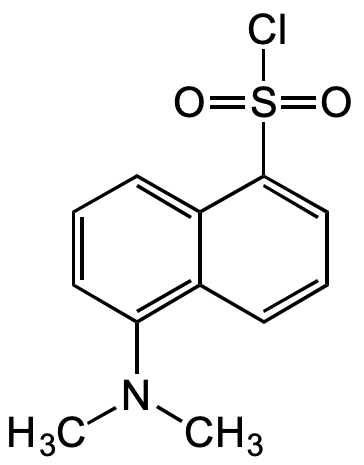
InChI=1S/C16H28N4O4S/c17-10(15(22)23)5-3-4-8-18-13(21)7-2-1-6-12-14-11(9-25-12)19-16(24)20-14/h10-12,14H,1-9,17H2,(H,18,21)(H,22,23)(H2,19,20,24)/t10?,11-,12-,14-/m0/s1
|