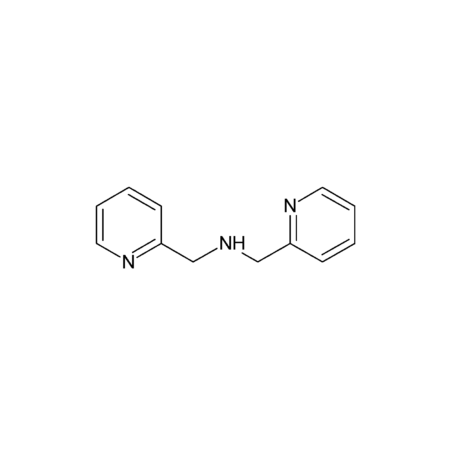
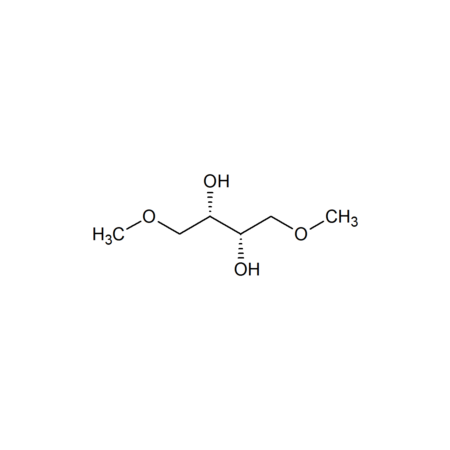
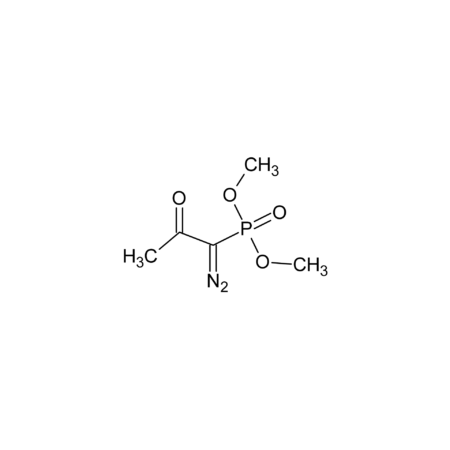
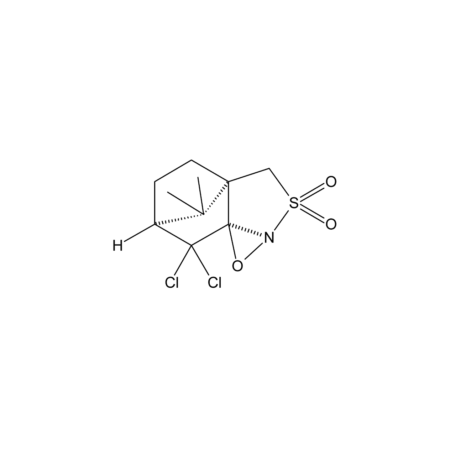
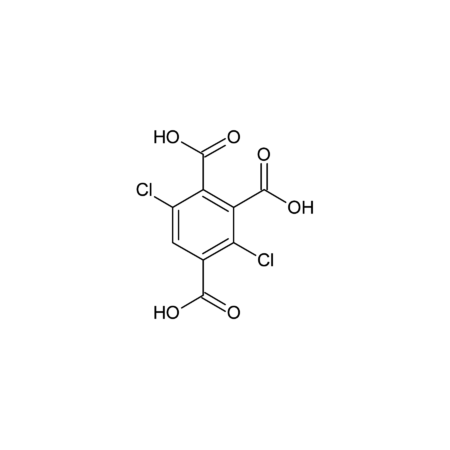
Specifications
- Purity
- ≥95% (GC)
- Appearance
- Yellow liquid
- Identity
- 1H-NMR
Properties
- Solvents
- water (slightly)
- Melting Point
- 107.28 °C (Predicted)
- Boiling Point
- 139-141 °C
- Refractive Index
- n20D 1.58 (lit.)
- Density
- 1.11 g/mL at 25 °C (lit.)
Downloads
- Safety Data Sheet
- CDX B0134 MSDS.pdf