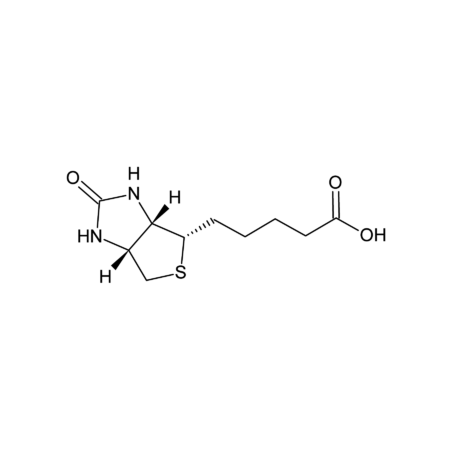
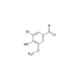
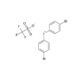
D-Biotin
- SKU
- B0148
Category: Vitamins
- Synonyms
- Bios II , Biotin , Coenzyme R , Vitamin B7 , Vitamin H , NSC 63865 , Factor S
- 58-85-5
- CAS-Number
- C10H16N2O3S
- Molecular Formula
- 244.3
- Molecular Weight
Specifications
- Purity
- ≥98% (NMR)
- Appearance
- White to off-white powder
- Identity
- 1H-NMR
Properties
- Solvents
- hot water, DMSO (49 mg/ml at 25 °C), alcohol, ethanol (<1 mg/ml at 25 °C), benzene
- Melting Point
- 233-236 °C
- Boiling Point
- ~573.6 °C at 760 mmHg (Predicted)
- Optical Activity
- α20/D +91º±2º, c = 2 in 0.1N NaOH
- Refractive Index
- n20D 1.55 (Predicted)
- Density
- ~1.3 g/cm3 (Predicted)
Downloads
- Safety Data Sheet
- CDX B0148 MSDS.pdf
- Shipping
- AMBIENT
- Short Term Storage
- +4°C
- Long Term Storage
- -20°C
- Handling Advice
- Protect from light and moisture.
- Use / Stability
- Stable for at least 2 years after receipt when stored at -20°C.
- Transportation
- Not dangerous goods
- Description
- D-(+)-Biotin, also known as vitamin H or coenzyme R, is a water-soluble B-vitamin (vitamin B7). D-(+)-Biotin is a cofactor responsible for carbon dioxide transfer in five carboxylase enzymes, such as acetyl-CoA carboxylase alpha, acetyl-CoA carboxylase beta, methylcrotonyl-CoA carboxylase, propionyl-CoA carboxylase or pyruvate carboxylase. It is important in fatty acid synthesis, branched-chain amino acid catabolism and gluconeogenesis. It covalently attaches to the epsilon-amino group of specific lysine residues in these carboxylases. This biotinylation reaction requires ATP and is catalyzed by holocarboxylase synthetase. Biotin is also covalently attached to distinct lysine residues in histones, affecting chromatin structure and mediating gene regulation. Biotin is widely used throughout the biotechnology industry to conjugate proteins for biochemical assays. Biotin's small size means the biological activity of the protein will most likely be unaffected. This process is called biotinylation. Because both streptavidin and avidin bind biotin with high affinity (Kd of 10-14 mol/l to 10-15 mol/l) and specificity, biotinylated proteins of interest can be isolated from a sample by exploiting this highly stable interaction. The sample is incubated with streptavidin/avidin beads, allowing capture of the biotinylated protein of interest. The attachment of biotin to various chemical sites is used to study various processes, including protein localization, protein interactions, DNA transcription and replication.
- Smiles
- OC(CCCC[C@H]1[C@](N2)([H])[C@](NC2=O)([H])CS1)=O
- InChi Key
- YBJHBAHKTGYVGT-ZKWXMUAHSA-N
- References
- (1) A. Chapman-Smith & J.E. Cronan Jr., J. Nutr. 129, 477S (1999) , (2) J. Zempleni & D.M. Mock, J. Nutr. Biochem. 10, 128 (1999) , (3) A. Holmberg, et al., Electrophoresis 26, 501 (2005) , (4) J. Zempleni, et al., Biofactors 35, 36 (2009)
- InChi
- InChI=1S/C10H16N2O3S/c13-8(14)4-2-1-3-7-9-6(5-16-7)11-10(15)12-9/h6-7,9H,1-5H2,(H,13,14)(H2,11,12,15)/t6-,7-,9-/m0/s1
D-(+)-Biotin, also known as vitamin H or coenzyme R, is a water-soluble B-vitamin (vitamin B7). D-(+)-Biotin is a cofactor responsible for carbon dioxide transfer in five carboxylase enzymes, such as acetyl-CoA carboxylase alpha, acetyl-CoA carboxylase beta, methylcrotonyl-CoA carboxylase, propionyl-CoA carboxylase or pyruvate carboxylase. It is important in fatty acid synthesis, branched-chain amino acid catabolism and gluconeogenesis. It covalently attaches to the epsilon-amino group of specific lysine residues in these carboxylases. This biotinylation reaction requires ATP and is catalyzed by holocarboxylase synthetase. Biotin is also covalently attached to distinct lysine residues in histones, affecting chromatin structure and mediating gene regulation. Biotin is widely used throughout the biotechnology industry to conjugate proteins for biochemical assays. Biotin’s small size means the biological activity of the protein will most likely be unaffected. This process is called biotinylation. Because both streptavidin and avidin bind biotin with high affinity (Kd of 10-14 mol/l to 10-15 mol/l) and specificity, biotinylated proteins of interest can be isolated from a sample by exploiting this highly stable interaction. The sample is incubated with streptavidin/avidin beads, allowing capture of the biotinylated protein of interest. The attachment of biotin to various chemical sites is used to study various processes, including protein localization, protein interactions, DNA transcription and replication.