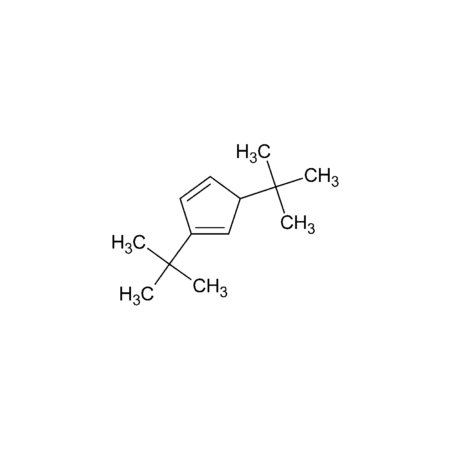
Di-tert-butylcyclopentadiene
- SKU
- B0136
Category: Building Blocks, Intermediates, Reagents
- Synonyms
- Di-tert-butyl-1,3-cyclopentadiene
- 120937-44-2
- CAS-Number
- C13H22
- Molecular Formula
- 178.31
- Molecular Weight
Specifications
- Purity
- ≥95% (GC)
- Appearance
- Transparent to yellow oil
- Identity
- 1H-NMR
Properties
- Solvents
- organic solvents
- Boiling Point
- 60-61°C / 4 torr
- Refractive Index
- n20/D 1.467
- Density
- 0.836 g/mL at 20 °C (lit.)
Downloads
- Safety Data Sheet
- CDX B0136 MSDS.pdf
- Shipping
- AMBIENT
- Short Term Storage
- +4°C
- Long Term Storage
- -20°C
- Handling Advice
- Protect from light and moisture.
- Use / Stability
- Stable for at least 2 years after receipt when stored at -20°C.
- Hazard statements
- H225-H315-H319-H335
- Precautionary statements
- P210-P261-P305 + P351 + P338
- GHS Symbol
- GHS02+GHS07
- Signal word
- Danger
- RIDADR
- UN3295 3
- Transportation
- Packing Group II
- Description
- Building block for synthesis. Frequently used as precursors to di-tert-butylcyclopentadienyl (Cpt) ligand in many sandwich and half sandwich complexes. Di-tert-butylcyclopentadiene reacts with excess potassium tert-butoxide in the presence of elemental selenium to yield cyclopentadienes bridged by two or three selenium atoms through one or two diselenoketal functionalities. It reacts with excess potassium tert-butoxide in the presence of elemental sulfur to yield cyclopentadienes bridged by sulfur atoms via one or two dithioketal functionalities.
- Smiles
- CC(C)(C)C1=CC(C(C)(C)C)C=C1
- InChi Key
- RADVPPBTEASJRZ-UHFFFAOYSA-N
- References
- (1) C.G. Venier & E.W. Casserly , JACS 112, 2808 (1990) , (2) G. Thaler, et al.: Eur. J. Inorg. Chem. 7, 973 (1998) , (3) A.V. Khvostov, et al.: Russ. Chem. Bull. 48, 2162 (1999) , (4) T.K. Hyster , e-EROS Encycl. Reag. Organ. Synth. (2015)
- InChi
- InChI=1S/C13H22/c1-12(2,3)10-7-8-11(9-10)13(4,5)6/h7-10H,1-6H3
Building block for synthesis. Frequently used as precursors to di-tert-butylcyclopentadienyl (Cpt) ligand in many sandwich and half sandwich complexes. Di-tert-butylcyclopentadiene reacts with excess potassium tert-butoxide in the presence of elemental selenium to yield cyclopentadienes bridged by two or three selenium atoms through one or two diselenoketal functionalities. It reacts with excess potassium tert-butoxide in the presence of elemental sulfur to yield cyclopentadienes bridged by sulfur atoms via one or two dithioketal functionalities.