Specifications
- Purity
- ≥98% (CE)
- Appearance
- White powder
- Identity
- 1H-NMR
Properties
- Solvents
- acetone, ethanol, DMSO
- Melting Point
- 140 - 145 °C
- Optical Activity
- [α]20/D -120±3°, c = 2% in acetic acid
Downloads
- Safety Data Sheet
- CDX B0224 MSDS.pdf
Category: Amino Acids, Peptides, Proteins
Amino acid protected by tert-butoxycarbonyl (Boc) group. Used as a reactant in peptide synthesis. Used to obtain asymmetric cystine peptides by enzymatic catalysis with immobilized papain. Used as educt for obtaining S-isoacyl dipeptide building blocks.
| Synonyms | Nα, Nα'-di-Boc-L-cystine, N,N'-Di(tert-butoxycarbonyl)-L-cystine, NSC 164046 |
|---|---|
| Purity | ≥98% (CE) |
| Appearance | White powder |
| CAS-Number | 10389-65-8 |
| Molecular Formula | C16H28N2O8S2 |
| Molecular Weight | 440.53 |
| Identity | 1H-NMR |
| Solvents | acetone, ethanol, DMSO |
| Melting Point | 140 – 145 °C |
| Optical Activity | [α]20/D -120±3°, c = 2% in acetic acid |
| Smiles | CC(C)(C)OC(N[C@H](C(O)=O)CSSC[C@@H](C(O)=O)NC(OC(C)(C)C)=O)=O |
| InChi Key | MHDQAZHYHAOTKR-UWVGGRQHSA-N |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use / Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Transportation | Not dangerous goods |
| References | (1) O. Keller, et al., Org. Synth. 63, 160 (1985), (2) D.-F. Tai, et al., Biotechnol. Lett. 15, 961 (1993), (3) D.-F. Tai, et al., Bioorg. Med. Chem. Lett. 5, 1475 (1995), (4) T. Yoshiya, et al., J. Pept. Sci. 14, 1203 (2008) |
| InChi | InChI=1S/C16H28N2O8S2/c1-15(2,3)25-13(23)17-9(11(19)20)7-27-28-8-10(12(21)22)18-14(24)26-16(4,5)6/h9-10H,7-8H2,1-6H3,(H,17,23)(H,18,24)(H,19,20)(H,21,22)/t9-,10-/m0/s1 |
| Quantity | 5 g, 25 g, Bulk |
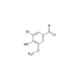
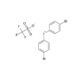 Bis(4-bromophenyl)iodonium triflate
Bis(4-bromophenyl)iodonium triflate