Specifications
- Purity
- ≥98% (HPLC)
- Appearance
- White to off-white powder
- Identity
- 1H-NMR
Properties
- Solvents
- DMSO, DMF, ethanol (all 20mg/ml), water (10mg/ml).
Category: Carbohydrates
Paeoniflorin has diverse cellular actions, including modulating NMDA and TRPV1 receptors. It is reported to inhibit testosterone synthesis and stimulate aromatase activity. It also reduces inflammatory signaling by inhibiting p38 MAP kinase and blocks pancreatic cancer cell apoptosis by suppressing MMP-2/9 and ERK signaling. It has been described as an adenosine A1 receptor activator with neuroprotective and antidepressant effects, as well as hypoglycemic activity. It can activate adenosine A-1 receptors to increase the translocations of PKC and GLUT4, two major signals for glucose uptake, from cytosol to membrane of the white adipocytes in rats. Recently it also was described as a liver X receptor (LXR) agonist, that can act as a ligand-activated transcription factor to exhibit antihyperlipidemic and neuroprotective effects. In addition, this potential anticancer agent, has ben described as a EP2 receptor agonists, a heat-shock protein (HSP)-inducing compound, by activation of HSF1, or by interacting with NOTCH1 or mTOR/HIF-1 signaling pathways.
| Synonyms | NSC 178886 |
|---|---|
| Purity | ≥98% (HPLC) |
| Appearance | White to off-white powder |
| CAS-Number | 23180-57-6 |
| Molecular Formula | C23H28O11 |
| Molecular Weight | 480.46 |
| Identity | 1H-NMR |
| Solvents | DMSO, DMF, ethanol (all 20mg/ml), water (10mg/ml). |
| Smiles | O[C@]12O[C@@]3([H])O[C@](C2)(C)[C@@]4([C@@]3(COC(C5=CC=CC=C5)=O)[C@@]1([H])C4)O[C@@H]([C@@H]([C@H]6O)O)O[C@H](CO)[C@H]6O |
| InChi Key | YKRGDOXKVOZESV-WRJNSLSBSA-N |
| Shipping | AMBIENT |
| Short Term Storage | RT |
| Long Term Storage | RT |
| Handling Advice | Protect from light and moisture. |
| Use / Stability | Stable for at least 2 years after receipt when stored at RT. |
| Transportation | Not dangerous goods |
| References | (1) J. Yamahara, et al., J. Pharmacobiodyn. 5, 921 (1982), (2) T. Takeuchi, et al., Amer. J. Chin. Med. 19, 73 (1991), (3) F.L. Hsu, et al., Planta Med. 63, 323 (1997), (4) C.W. Lai, et al., Life Sci. 62, 1591 (1998), (5) H.O. Yang, et al., Fitoterapia 75, 45 (2004), (6) D. Yan, et al., Cell Stress Chaperones 9, 378 (2004), (7) D.Z. Liu, et al., Br. J. Pharmacol. 146, 604 (2005), (8) H.Q. Liu, et al., Br. J. Pharmacol. 148, 314 (2006), (9) J.Y. Hung, et al., Clin. Exp. Pharmacol. Physiol. 35, 141 (2008), (10) H. Wu, et al., Biomed. Pharmacother. 62, 659 (2008), (11) J. Fu, et al., Comp. Med. 59, 557 (2009), (12) J. Huang, et al., Int. Immunopharmacol. 10, 1279 (2010), (13) Q.Q. Mao, et al., Phytother. Res. 25, 681 (2011), (14) S. Hu, et al., Anticancer Drugs 24, 140 (2013), (15) R.B. Guo, et al., PLoS One 7, e49701 (2012), (16) H.R. Lin, J. Asian Nat. Prod. Res. 15, 35 (2013), (17) F.M. Qiu, et al., Neurosci. Lett. 541, 209 (2013), (18) W. Li, et al., Mol. Med. Rep. 12, 2735 (2015), (19) F. Han, et al., Cell Biosci. 6, 37 (2016), (20) S. Wang & W. Liu, Mol. Med. Rep. 14, 2143 (2016), (21) R. Lu, et al., Biomed. Pharmacother. 96, 137 (2017), (22) J. Zhang, et al., Mol. Med. Rep. 17, 1321 (2018), (23) H. Wang, et al., J. Cell Physiol. (Epub ahead of print) (2018), (24) L.B. Jin, et al., Mol. Med. Rep. 17, 5095 (2018) |
| Quantity | 25 mg, 100 mg, 250 mg, Bulk |
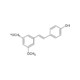
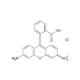 Rhodamine 110 chloride
Rhodamine 110 chloride